▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk for the UK and hpra.ie/homepage/about-us/report-an-issue for Republic of Ireland. Adverse events should also be reported to UCB Pharma Ltd at ucbcares.uk@ucb.com or 0800 2793177 for the UK and UCB (Pharma) Ireland Ltd at ucbcares.ie@ucb.com or 1800 930075 for Republic of Ireland.

BIMZELX DELIVERED HIGH TREATMENT TARGETS IN PsA 1-4
BIMZELX® (bimekizumab) is indicated for the treatment of: active PsA, alone or in combination with methotrexate, in adults who have had an inadequate response or who have been intolerant to one or more DMARDs; active nr-axSpA, in adults with objective signs of inflammation as indicated by elevated CRP and/or MRI, who have responded inadequately or are intolerant to NSAIDs; and active AS, in adults who have responded inadequately or are intolerant to conventional therapy.3
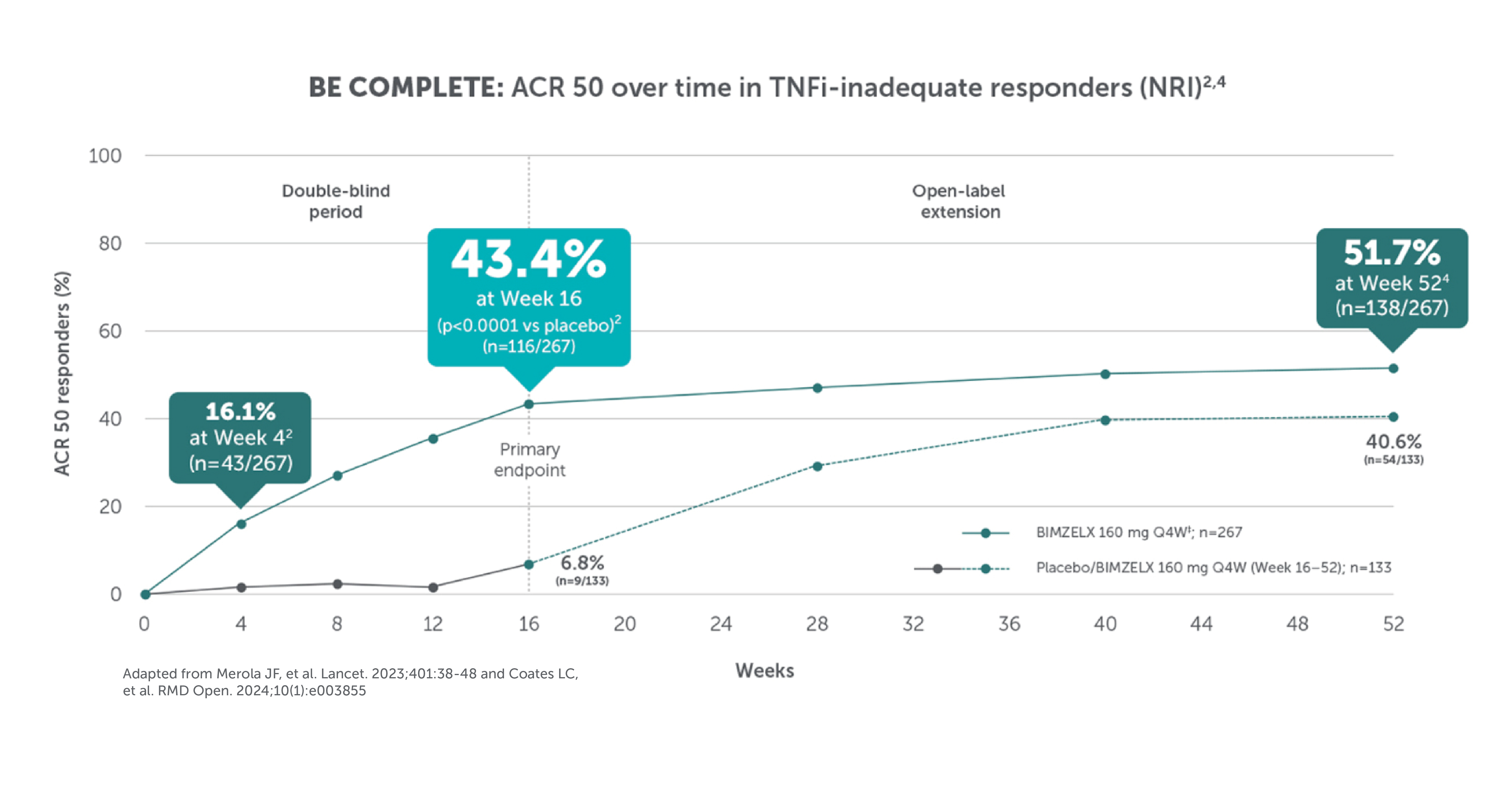
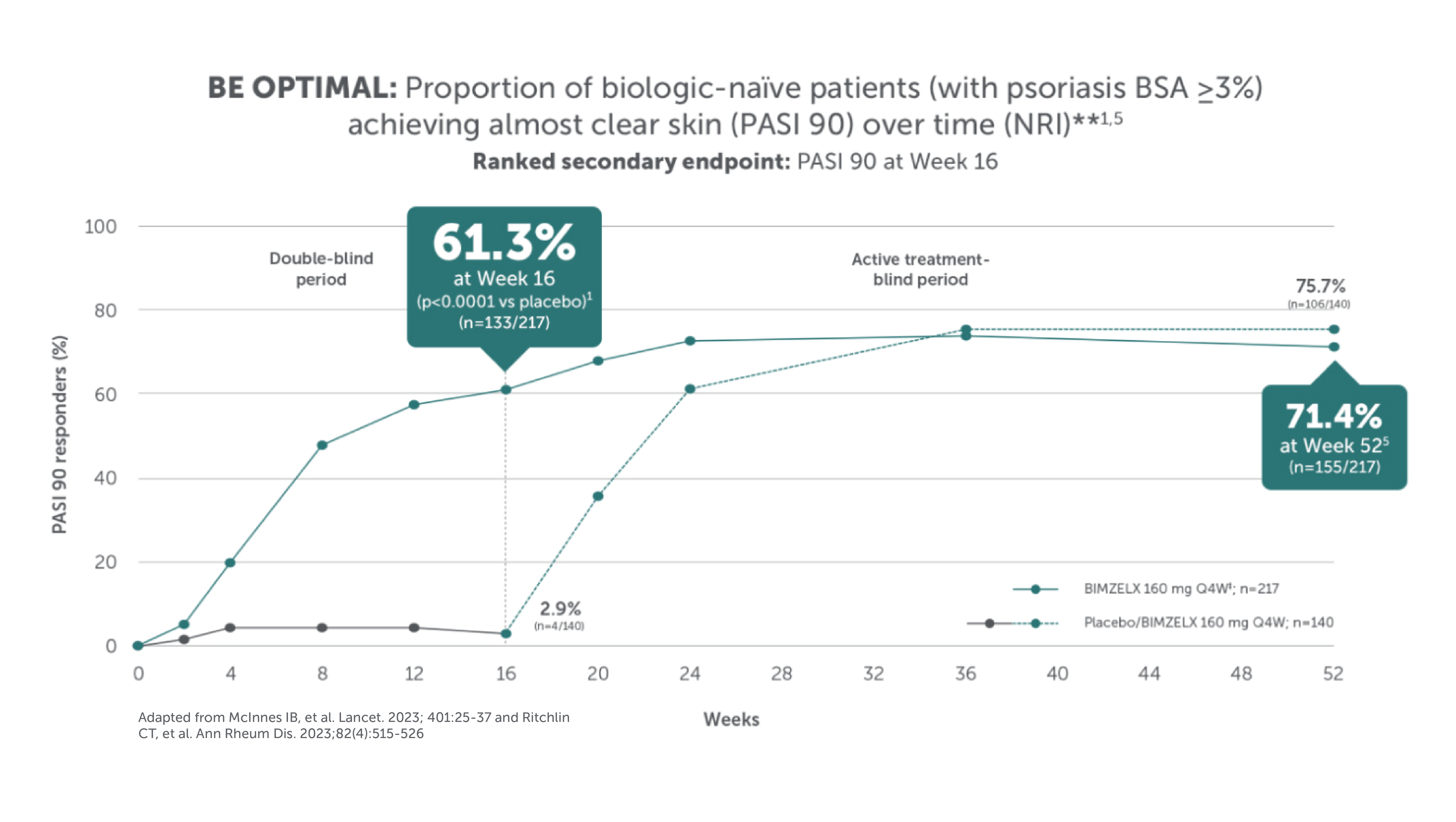
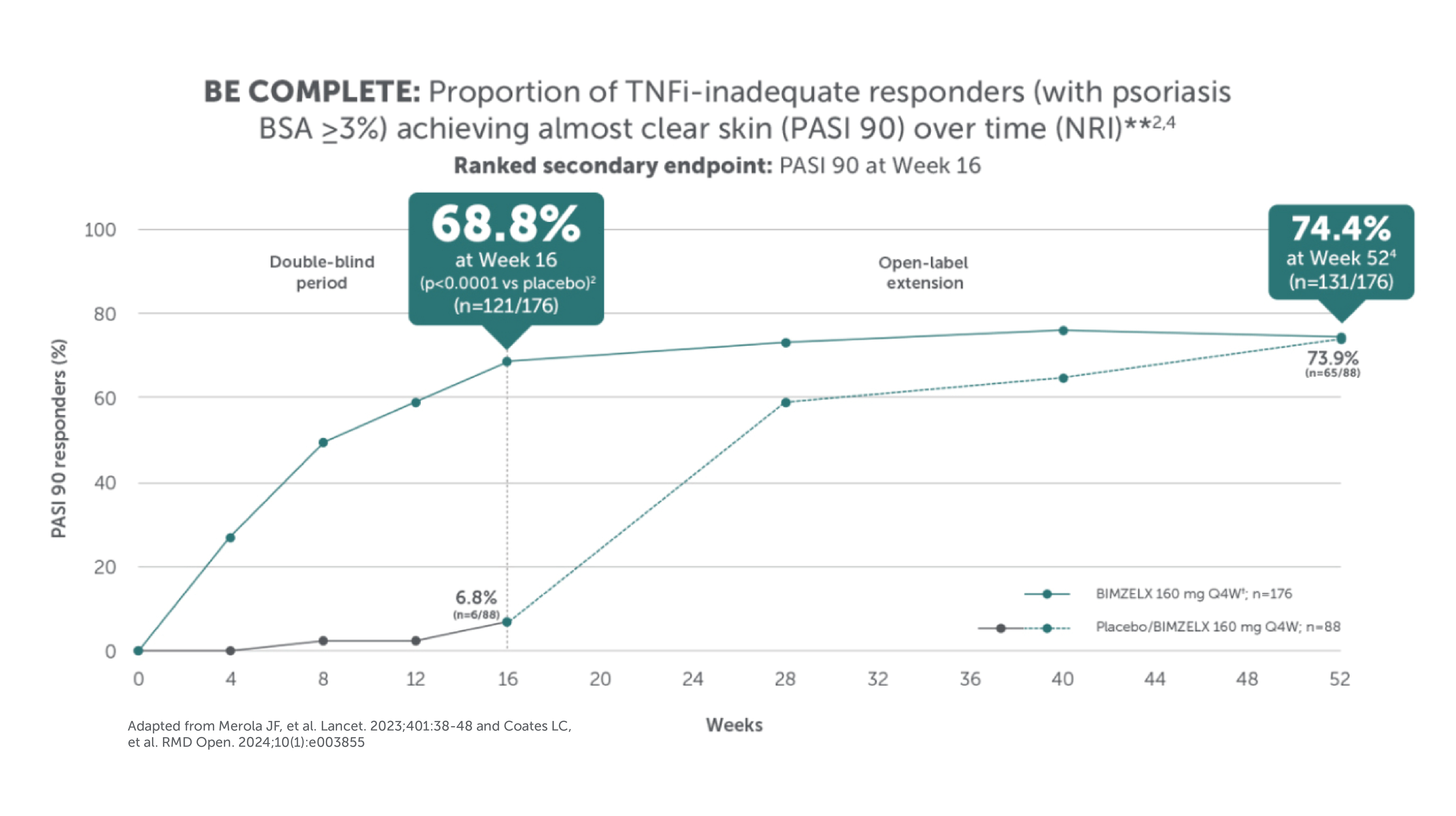
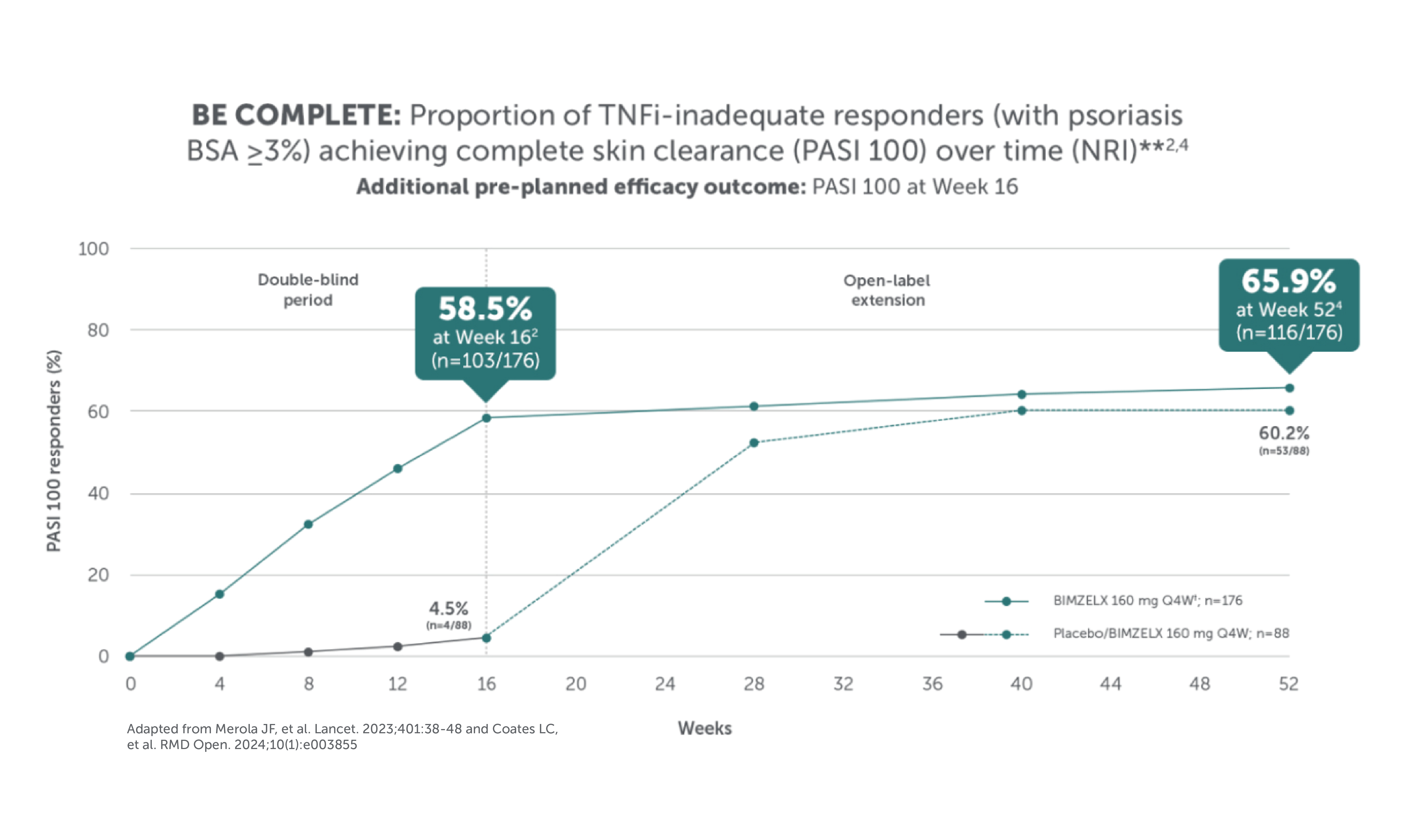
BIMZELX DELIVERED EFFICACY, SUSTAINED OVER TIME IN PATIENTS WITH PsA (TO WEEK 52) 1–4
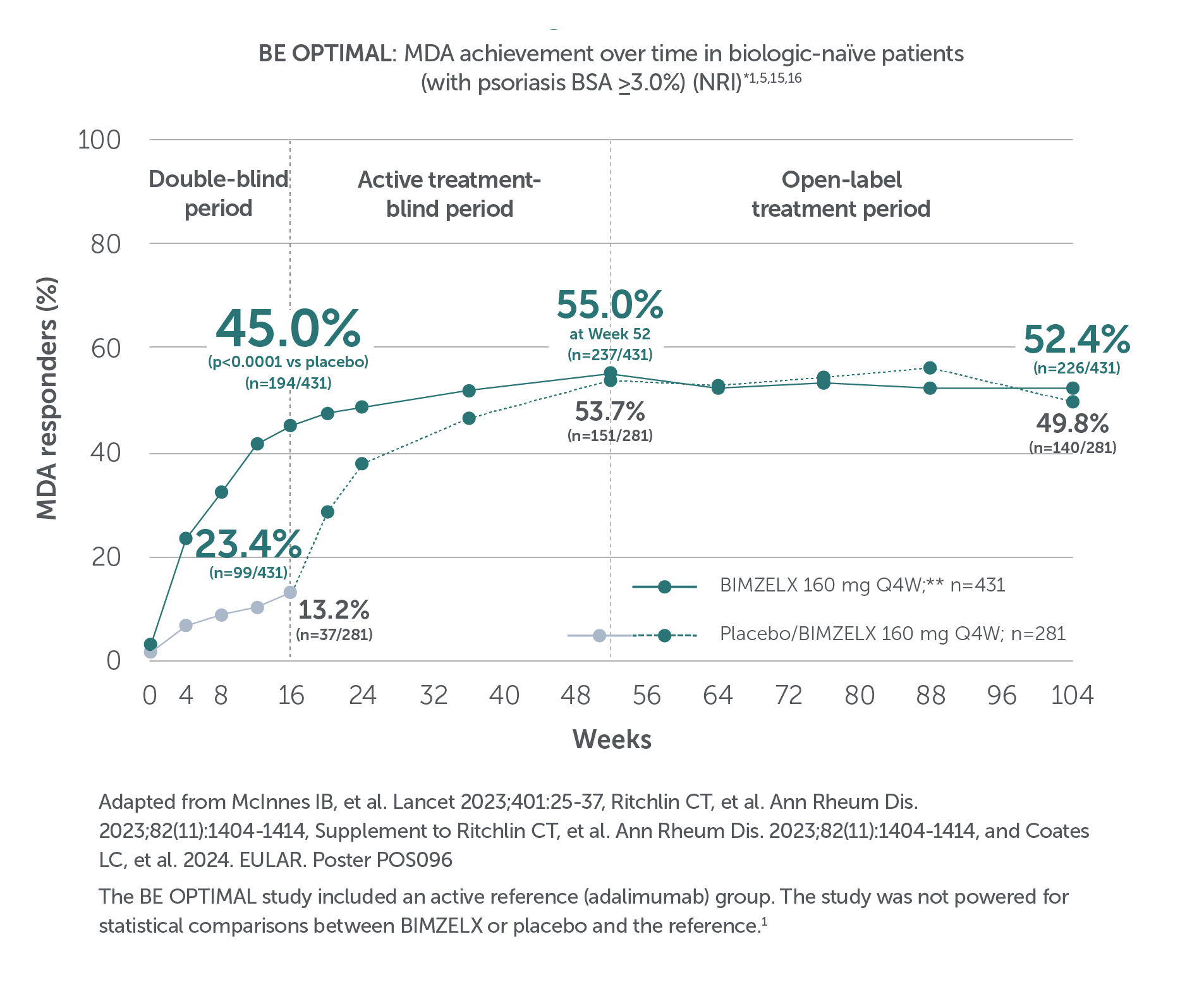
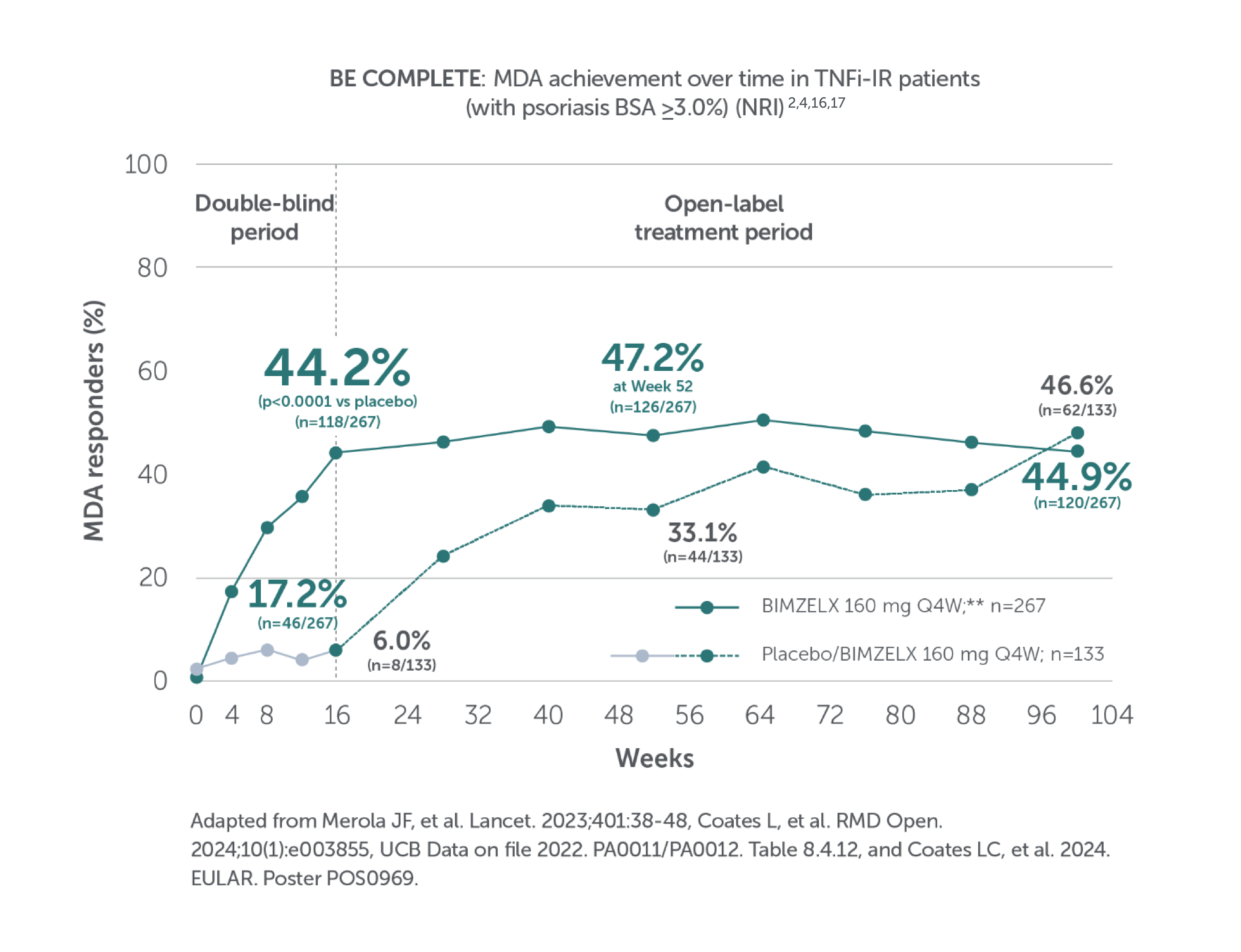
In two pivotal phase III trials, 43.9% (n=189/431) of biologic-naïve and 43.4% (n=116/267) of TNFi-inadequate responder patients achieved the primary endpoint of ACR 50 at Week 16 with BIMZELX (versus 10.0% [n=28/281] and 6.8% [n=9/133] with placebo, respectively; (p<0.0001 and p<0.001 for each trial respectively)1-3

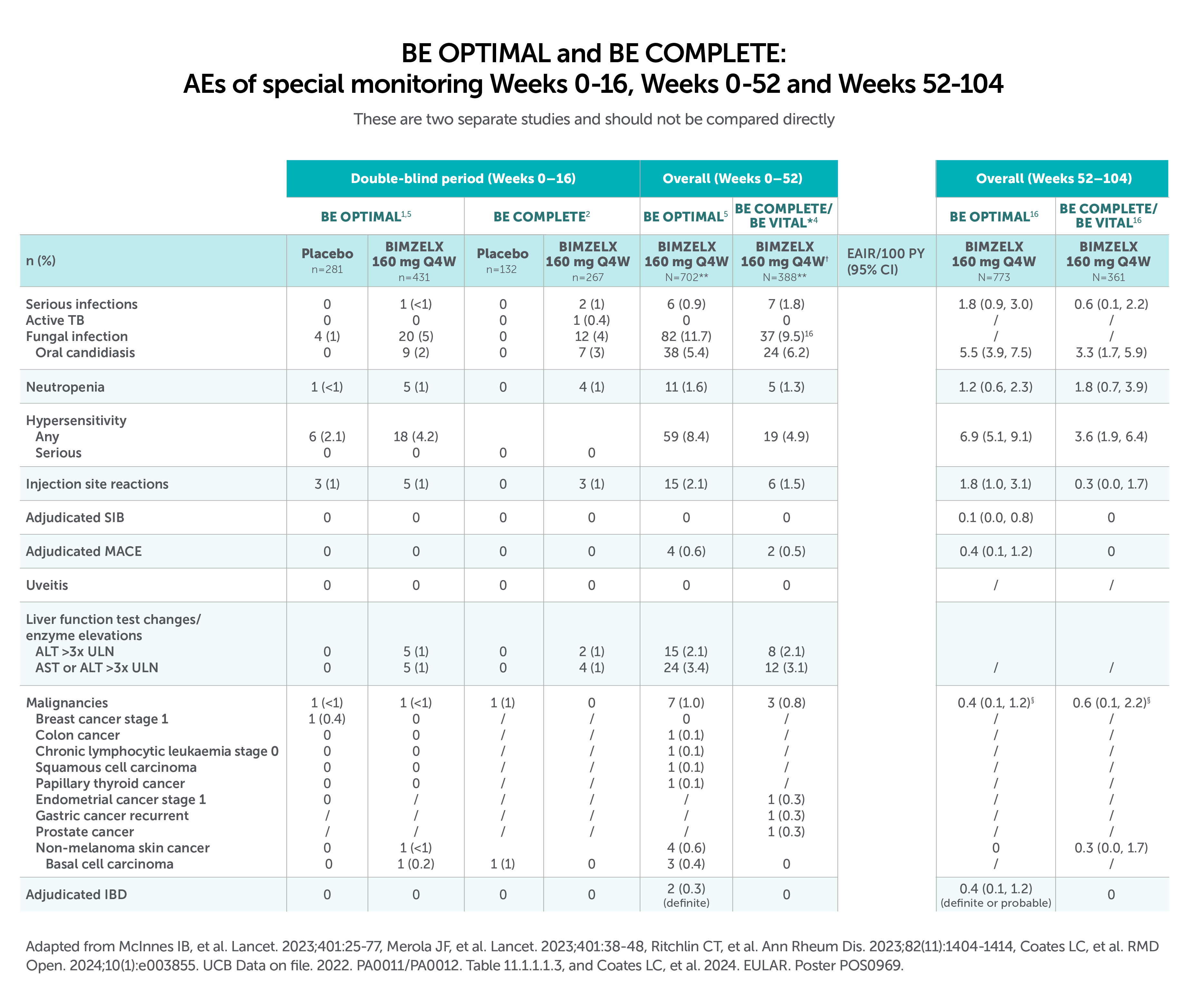
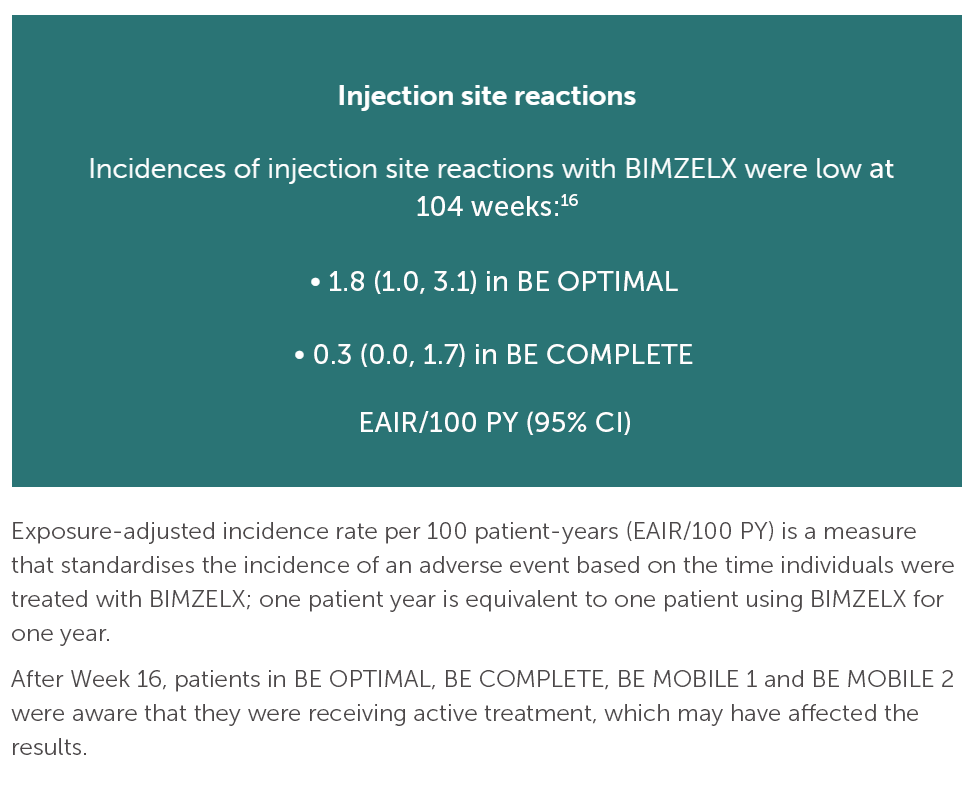
After Week 16, patients in BE OPTIMAL, BE COMPLETE, BE MOBILE 1 and BE MOBILE 2 were aware that they were receiving active treatment, which may have affected the results. *Week 52 data are from patients who completed BE COMPLETE and entered the BE VITAL open-label extension study.4 Week 52 data are from BE MOBILE 1 and BE MOBILE 2 and are the open-label maintenance phase of the studies.9 **Includes patients who switched from BIMZELX Q4W (events reports after the switch only).5,16 †One death occurred in a patient receiving BIMZELX due to a motorcycle accident.5 ‡One sudden death occurred in a patient with a history of cardiac events.4 ¥One death due to acute myocardial infarction, reported as unrelated to the study drug.16 §Malignancies excluding non-melanoma skin cancer.16
BIMZELX DOSING FOR PATIENTS WITH PsA3
HOW TO USE
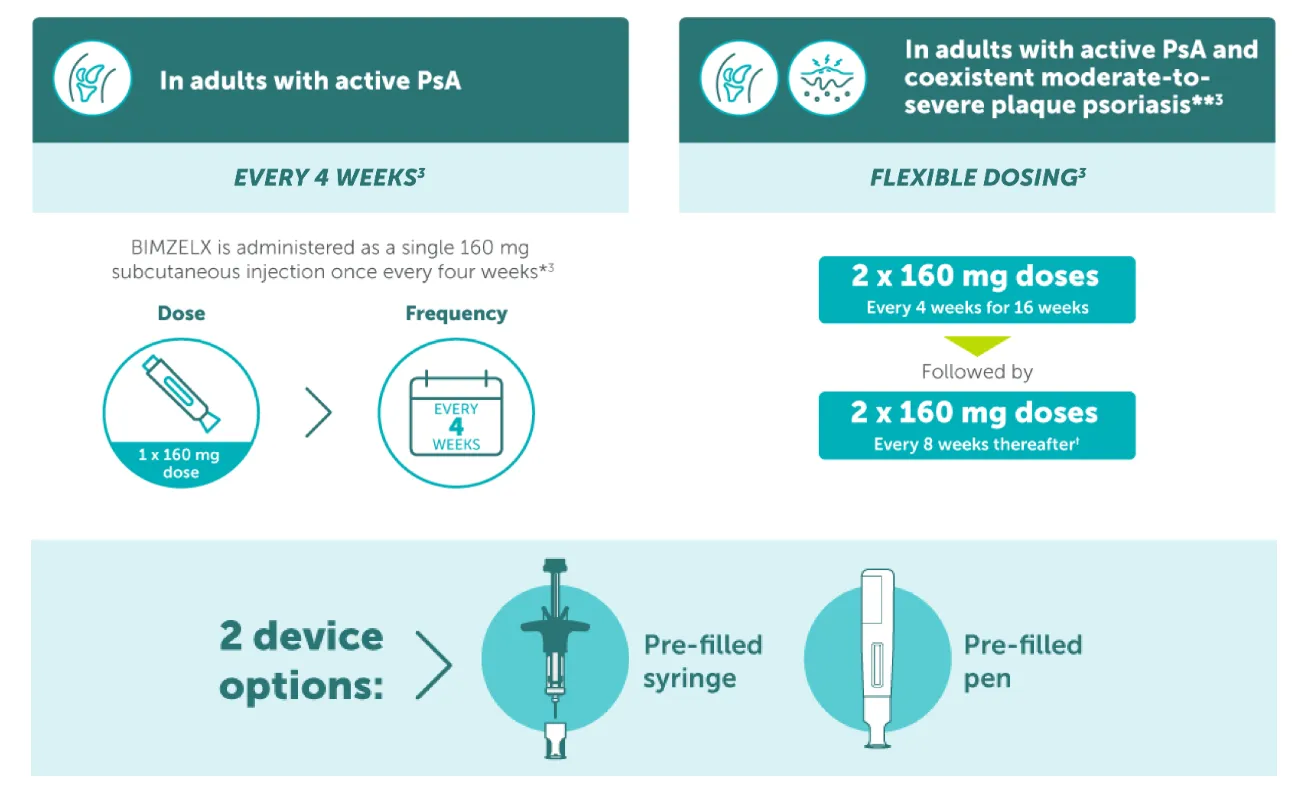
* The recommended dose for adult patients with active psoriatic arthritis is 160 mg (given as one subcutaneous injection of 160 mg) every 4 weeks. Consideration should be given to discontinuing treatment in patients who have shown no improvement by 16 weeks of treatment;3 **The recommended dose for adult patients with psoriatic arthritis and coexistent moderate-to-severe plaque psoriasis is the same as for plaque psoriasis, 320 mg (given as two subcutaneous injections of 160 mg each) at Week 0, 4, 8, 12, 16, and every 8 weeks thereafter. Consideration should be given to discontinuing treatment in patients who have shown no improvement by 16 weeks of treatment.3 †If a sufficient clinical responsed in the joints cannot be maintained after Week 16, a switch to 160 mg Q4W can be considered.3
Pivotal phase III study design: BE OPTIMAL
Biologic-naïve patients with active PsA21
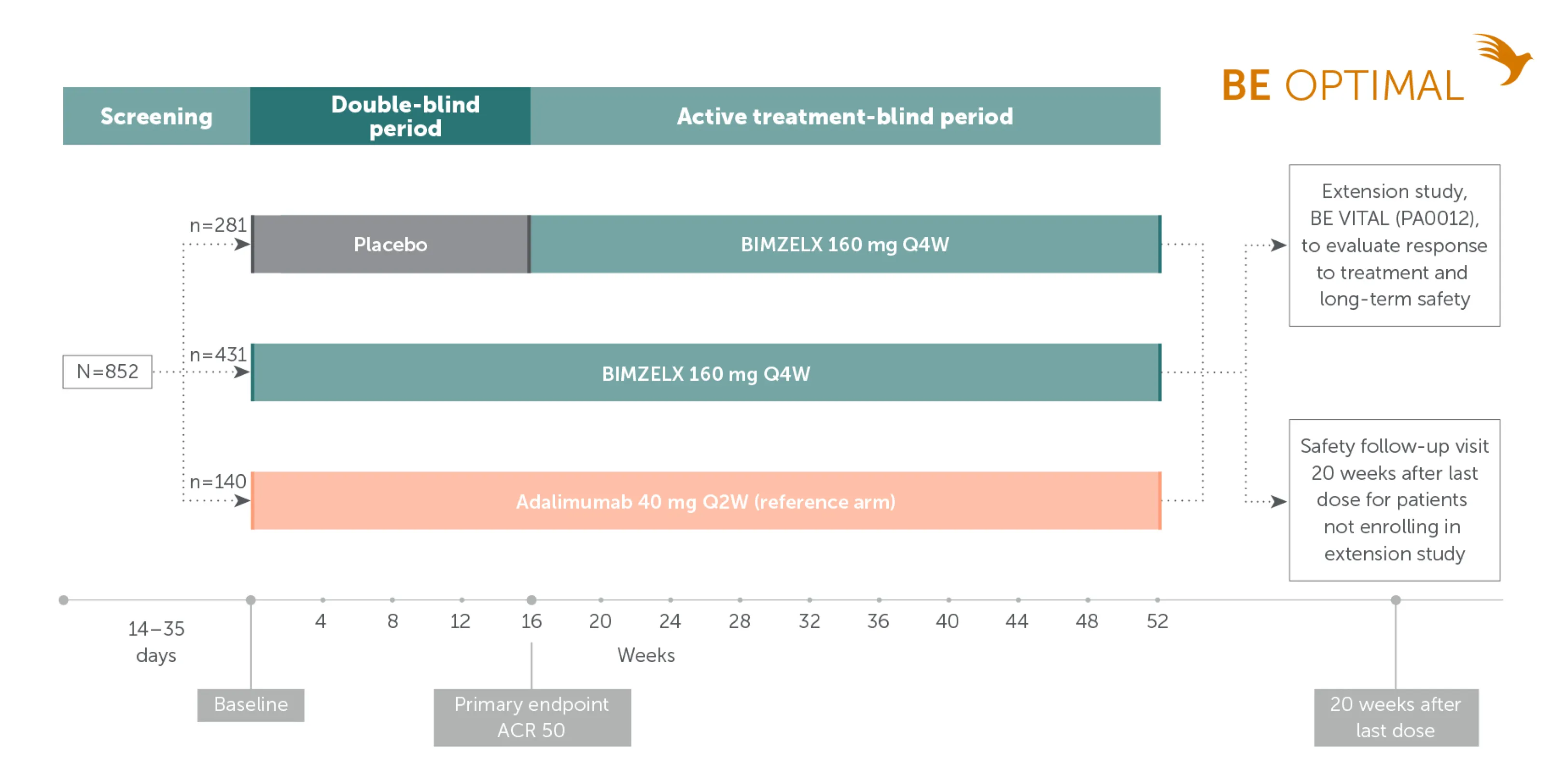
Adapted from McInnes IB, et al. Lancet. 2023;401:25-77. Supplementary appendix.
Pivotal phase III study design: BE COMPLETE
TNFi-inadequate responder patients with active PsA22
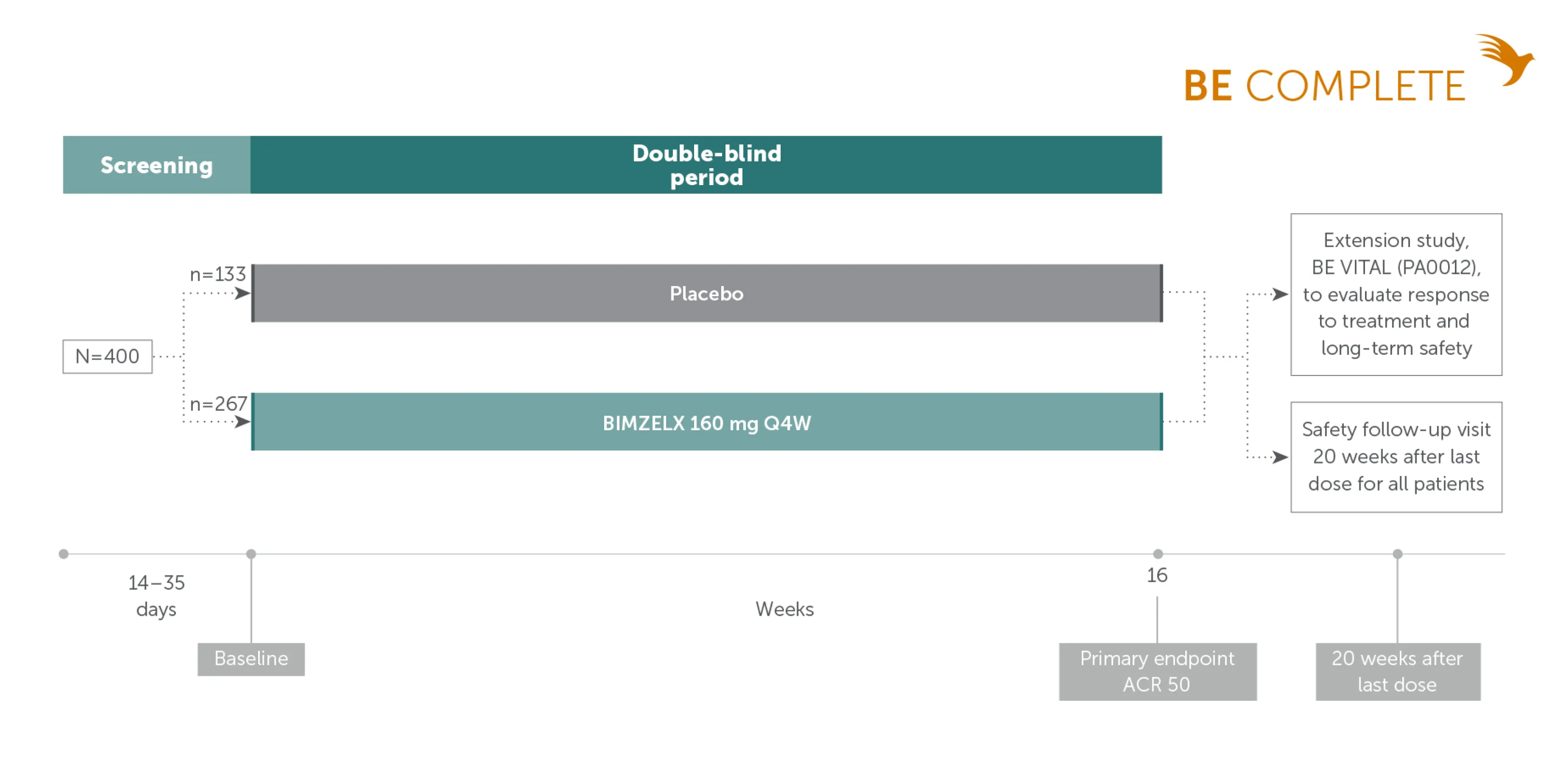
Adapted from Merola JF, et al. Lancet. 2023;401:38-48. Supplementary appendix.
Phase IIb and open-label extension study design: BE ACTIVE23
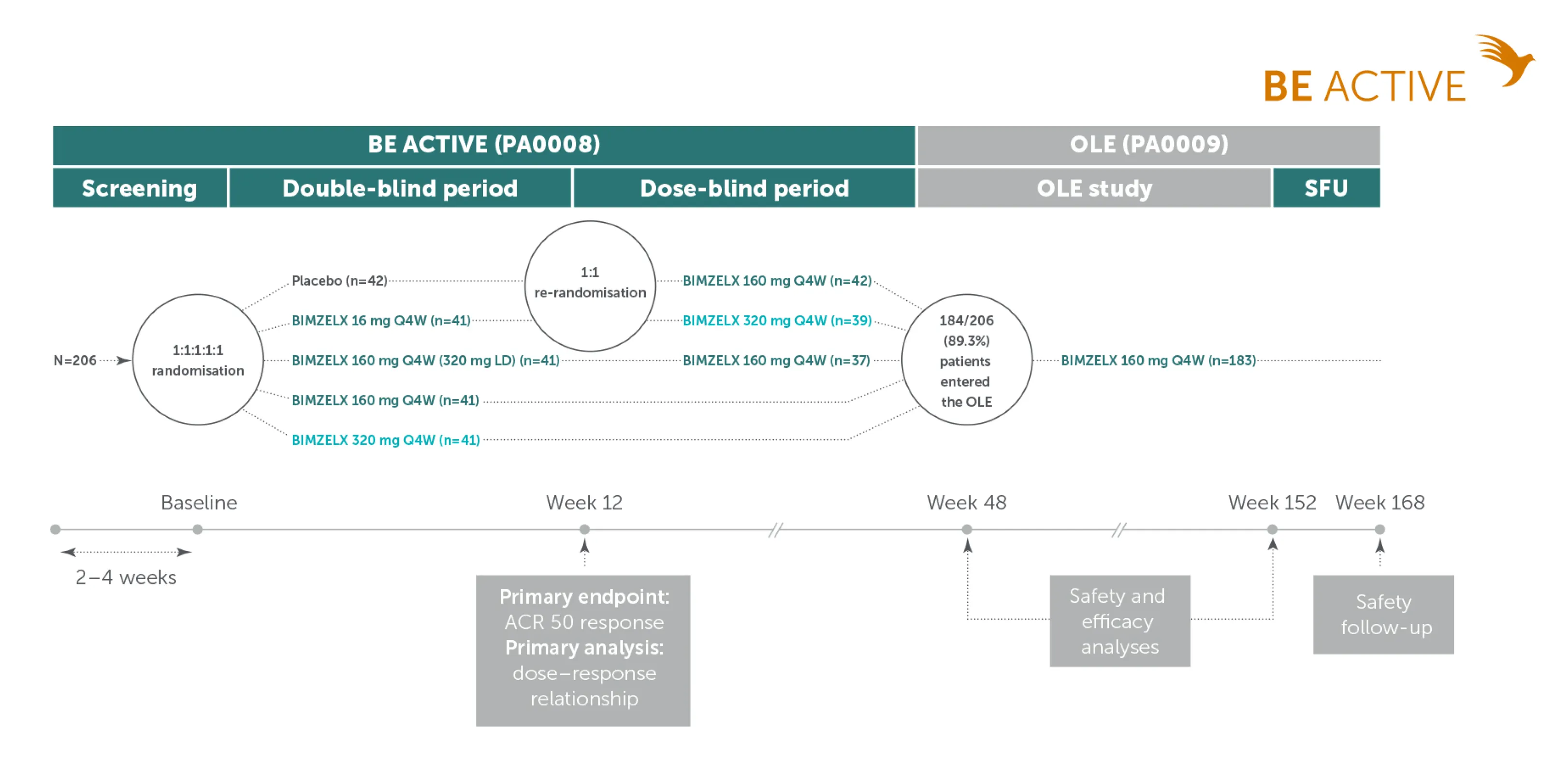
Adapted from Coates LC. et al. Arthritis Rheumatol. 2022;74:1959-1970. Supplementary appendix

IE-BK-2400114
Date of creation: November 2024