▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare Professionals are asked to report any suspected adverse reactions. Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk for the UK and hpra.ie/homepage/about-us/report-an-issue for Ireland Or, via the MHRA Yellow Card App in the Google Play or Apple App Store. Adverse events should also be reported to UCB Pharma Limited at UCBCares.UK@ucb.com or 0800 2793177.

BIMZELX DELIVERED HIGH TREATMENT TARGETS ACROSS THE FULL axSpA SPECTRUM1,2
BIMZELX® (bimekizumab) is indicated for the treatment of: active PsA, alone or in combination with methotrexate, in adults who have had an inadequate response or who have been intolerant to one or more DMARDs; active nr-axSpA, in adults with objective signs of inflammation as indicated by elevated CRP and/or MRI, who have responded inadequately or are intolerant to NSAIDs; and active AS, in adults who have responded inadequately or are intolerant to conventional therapy.2
EFFICACY
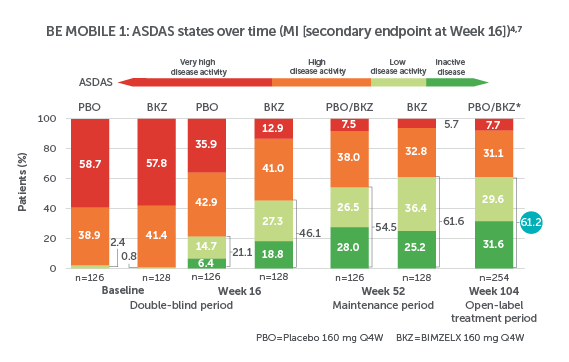
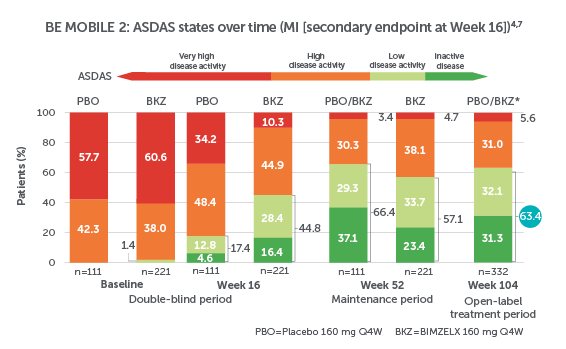
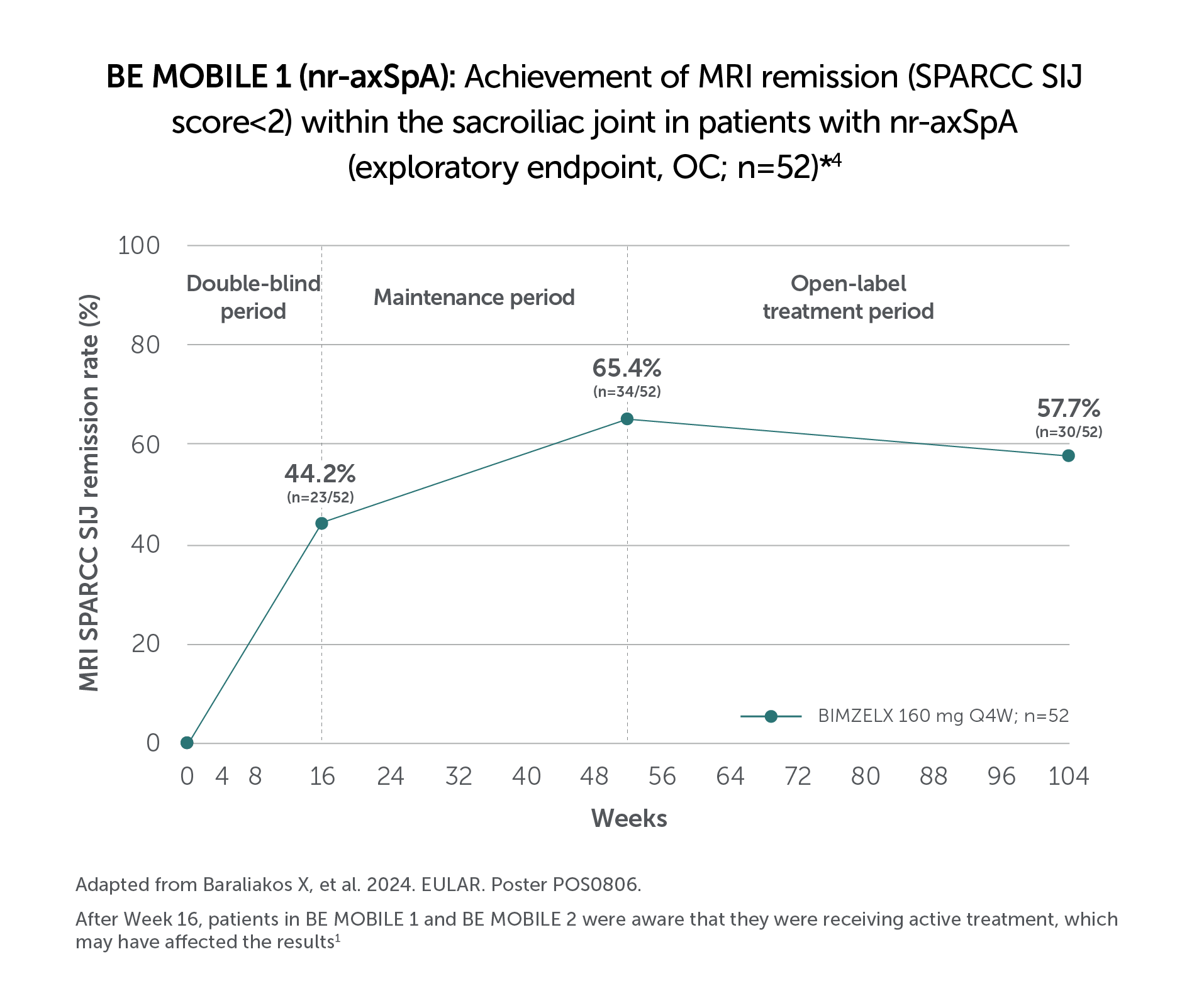
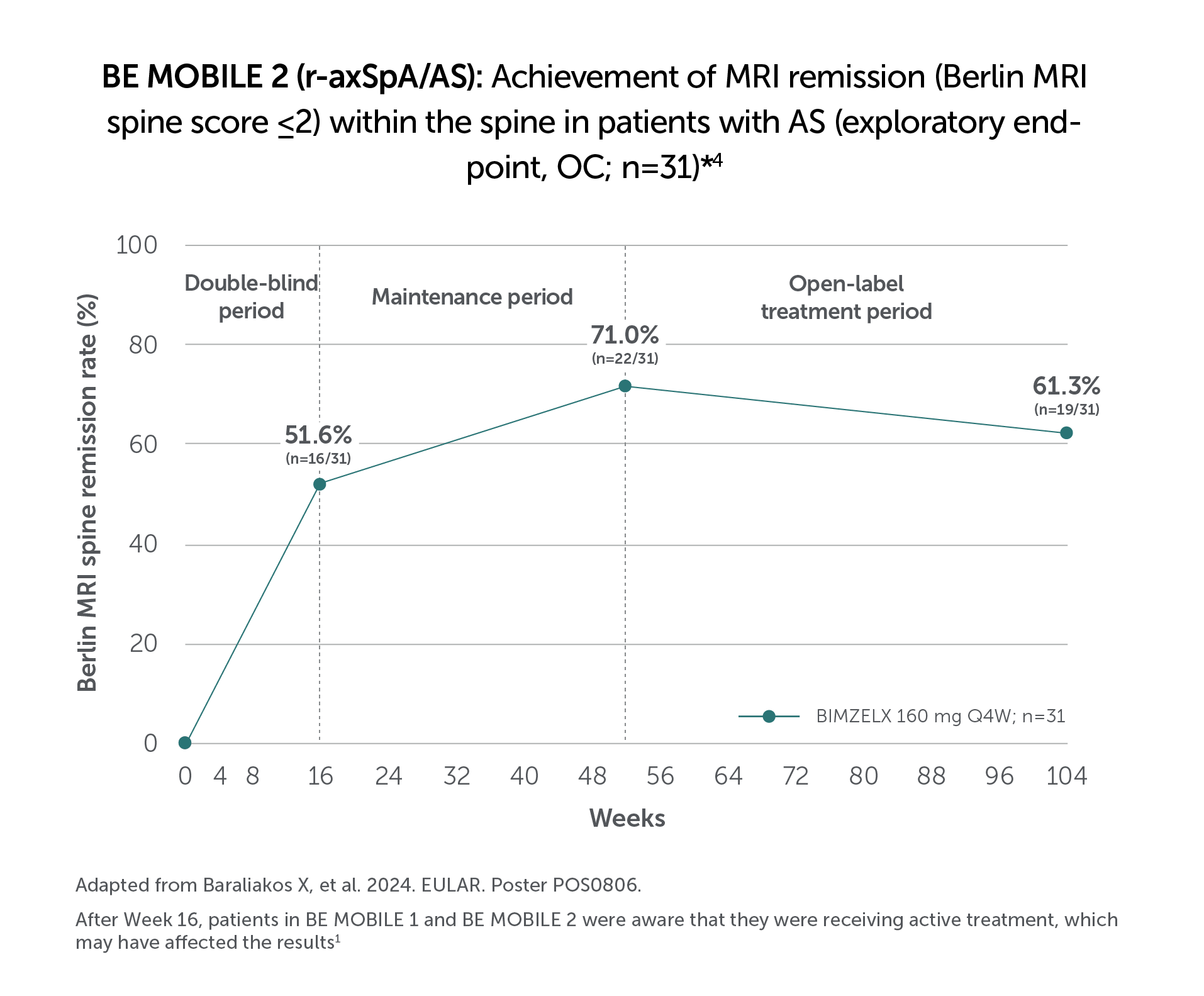
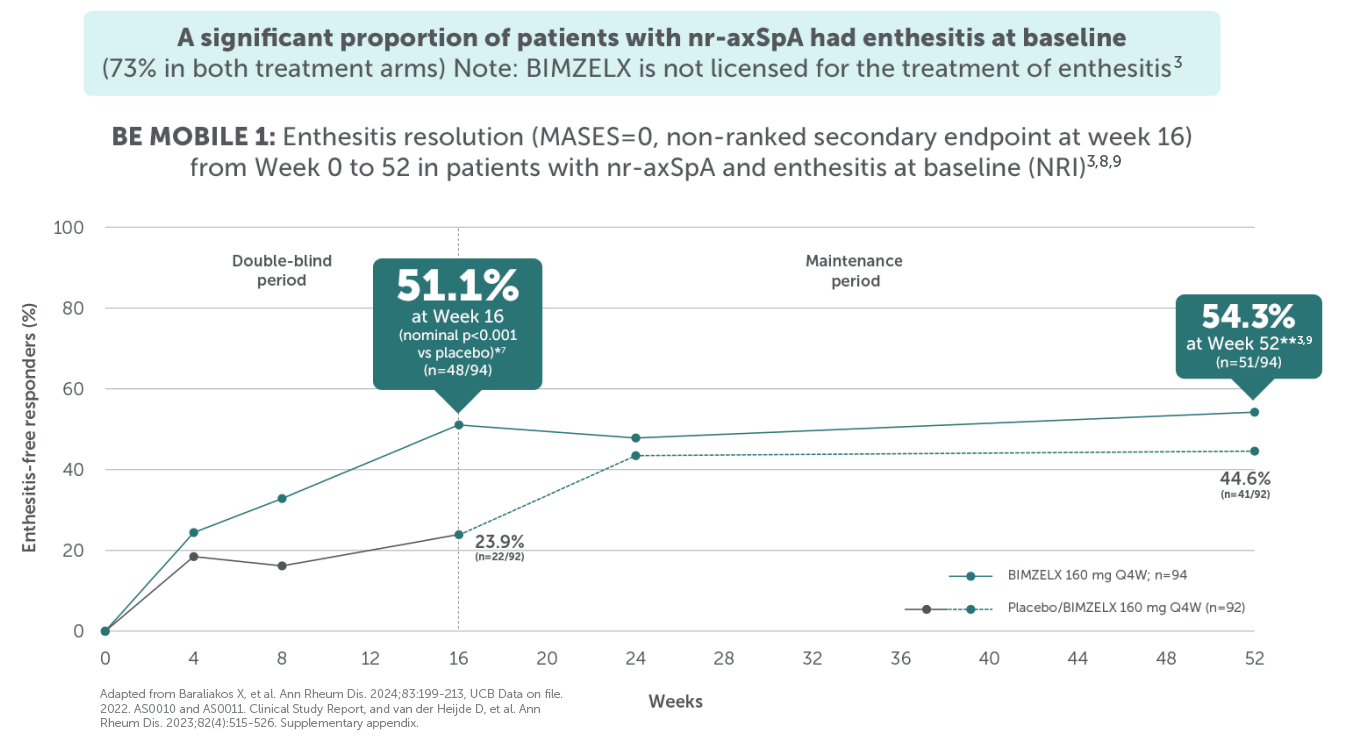
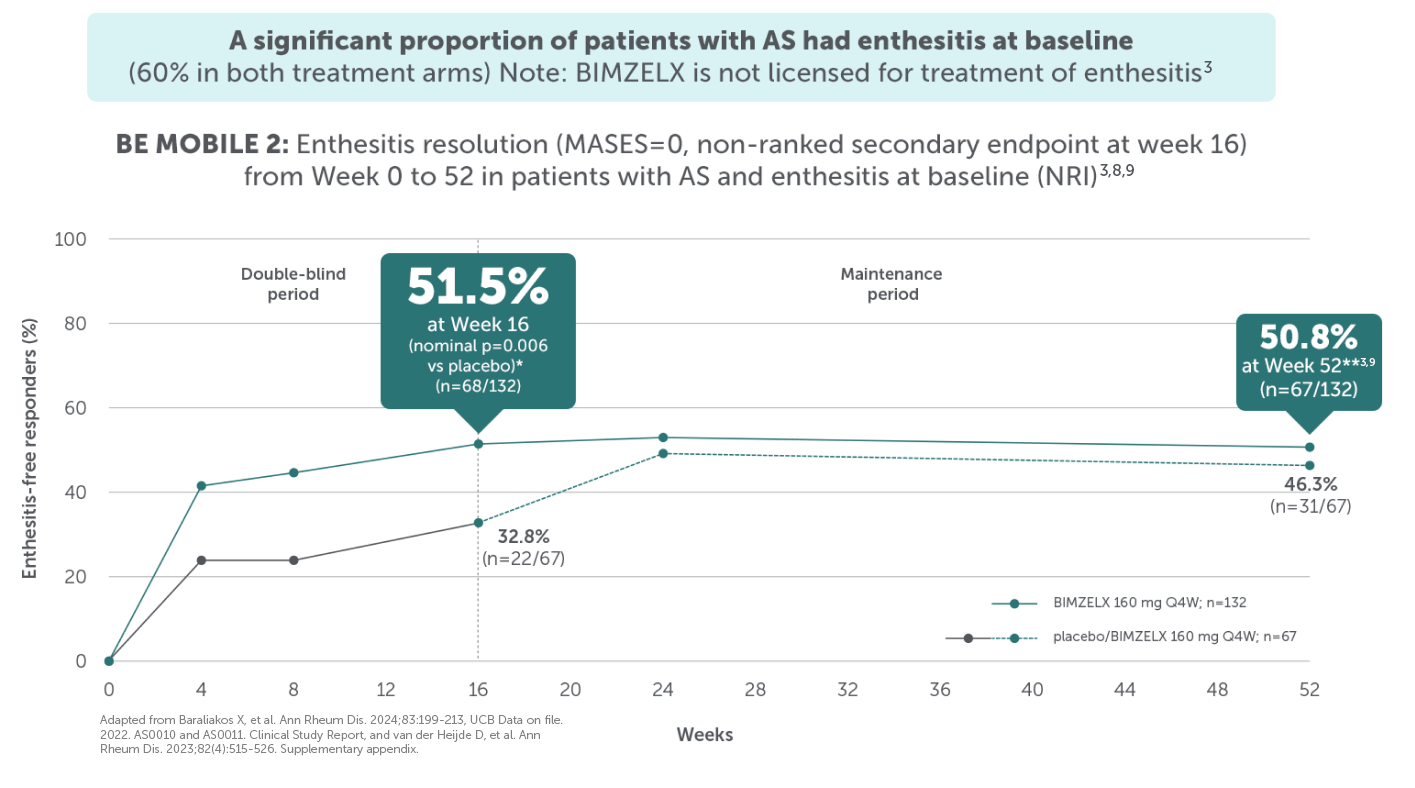
BIMZELX DELIVERED EFFICACY IN AXSPA PATIENTS, SUSTAINED OVER TIME (TO WEEK 104)4
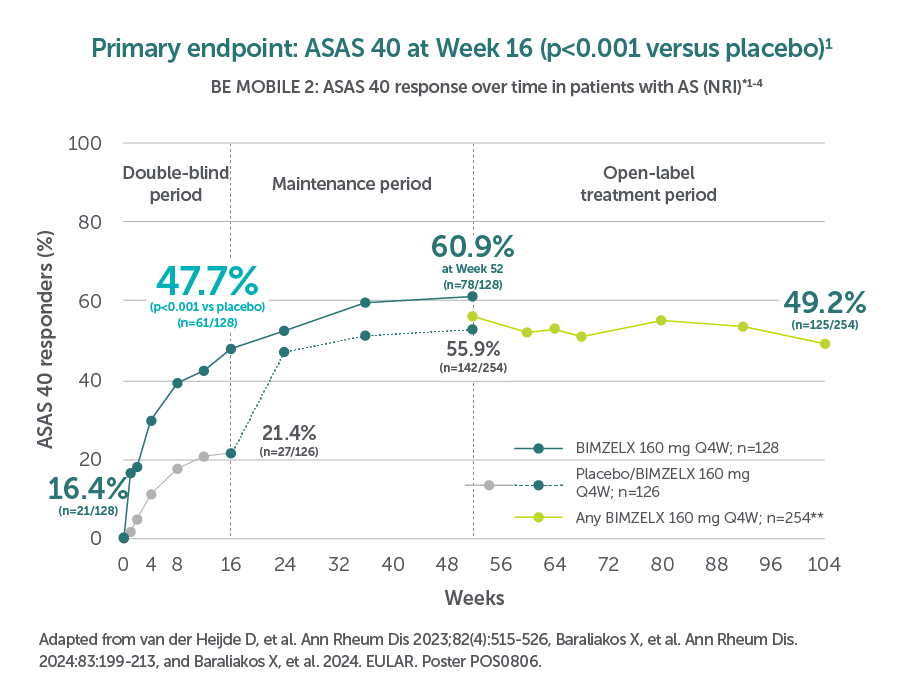
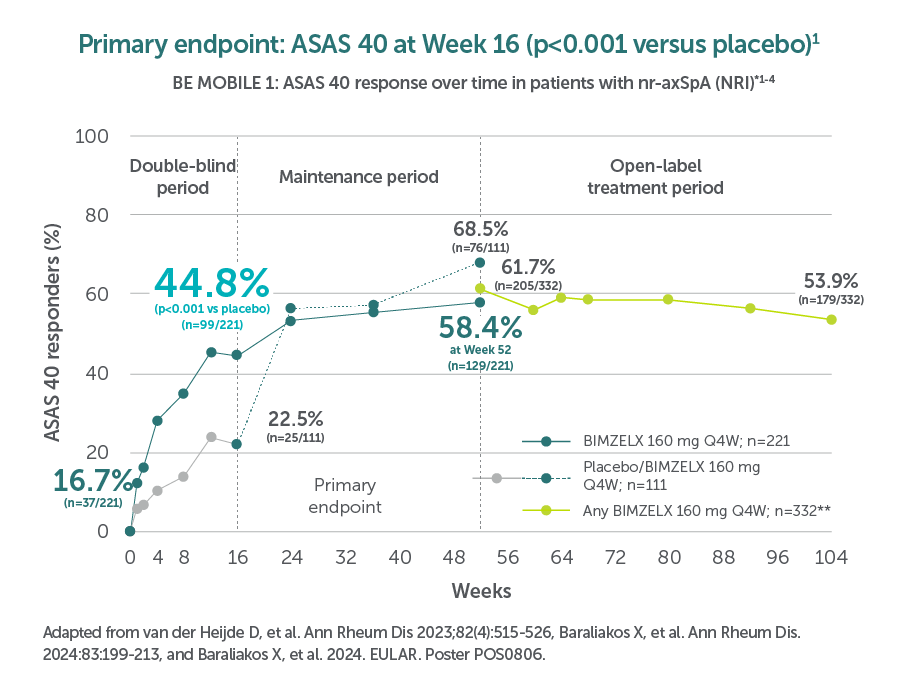
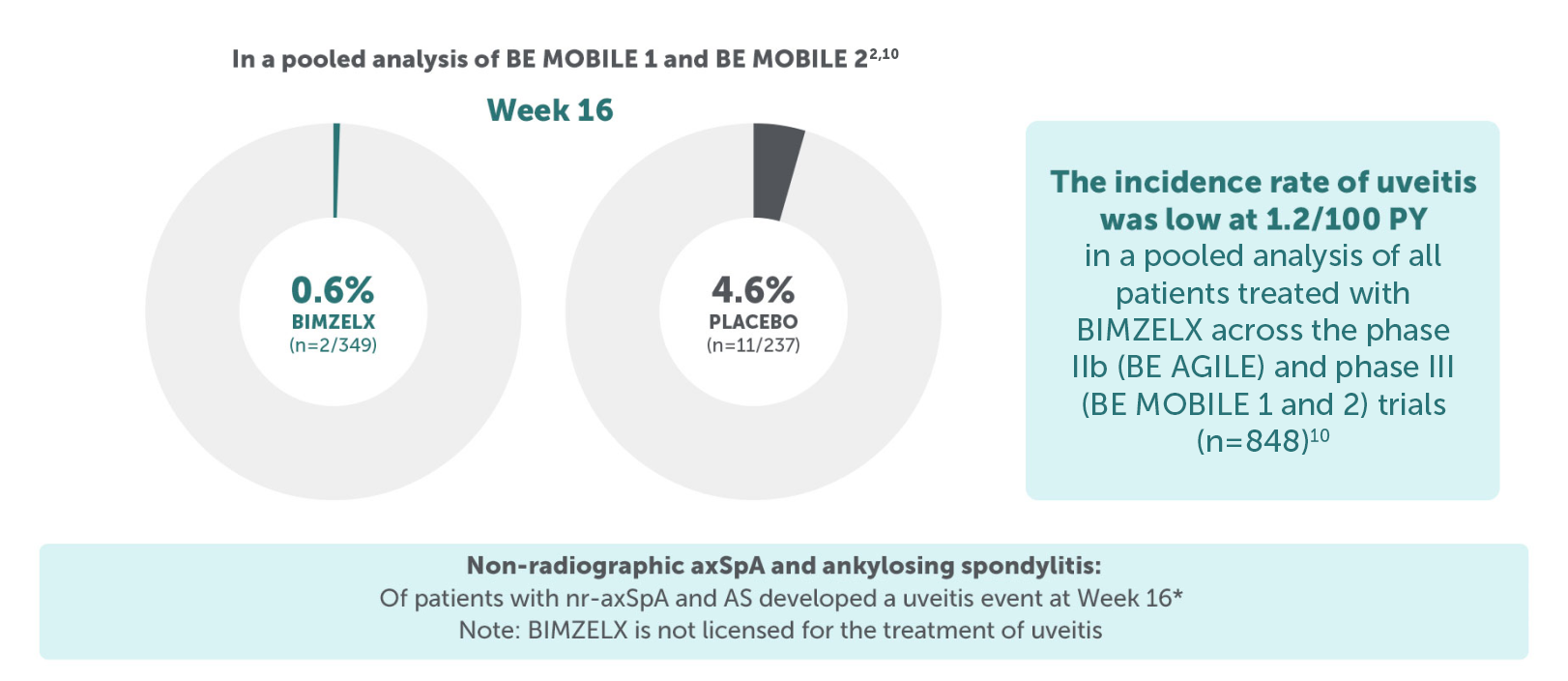
In two pivotal phase Ill trials, 47.7% (n=61/128) of patients with nr-axSpA and 44.8% (n=99/221) of patients with AS achieved the primary endpoint of ASAS 40 at Week 16 with BIMZELX (vs 21.4% [n=27/126] and 22.5% [n=25/111] with placebo, respectively; p<0.001 in both trials)1,2
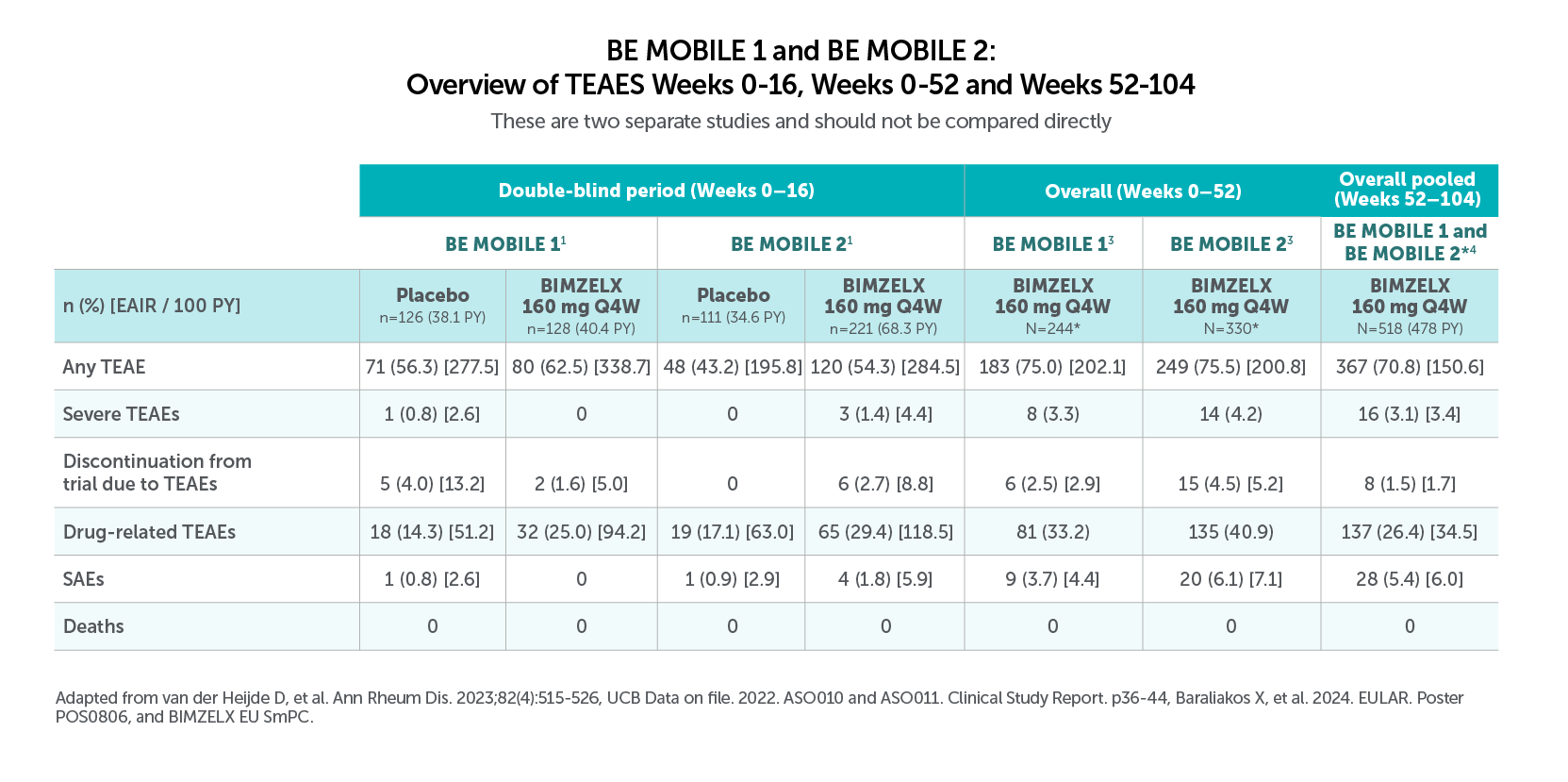
Most common TEAES (>5.0% in BE MOBILE 1 and BE MOBILE 2) with BIMZELX across 52 weeks were:
• Nasopharyngitis (12.3%, n=30/244, and 9.1%, n=30/330), oral candidiasis (7.4%, n=18/244, and 6.1%, n=20/330), and URTIS (9.4%, n=23/244, and 6.4%, n=21/330) in BE MOBILE 1 and BE MOBILE 2, respectively3
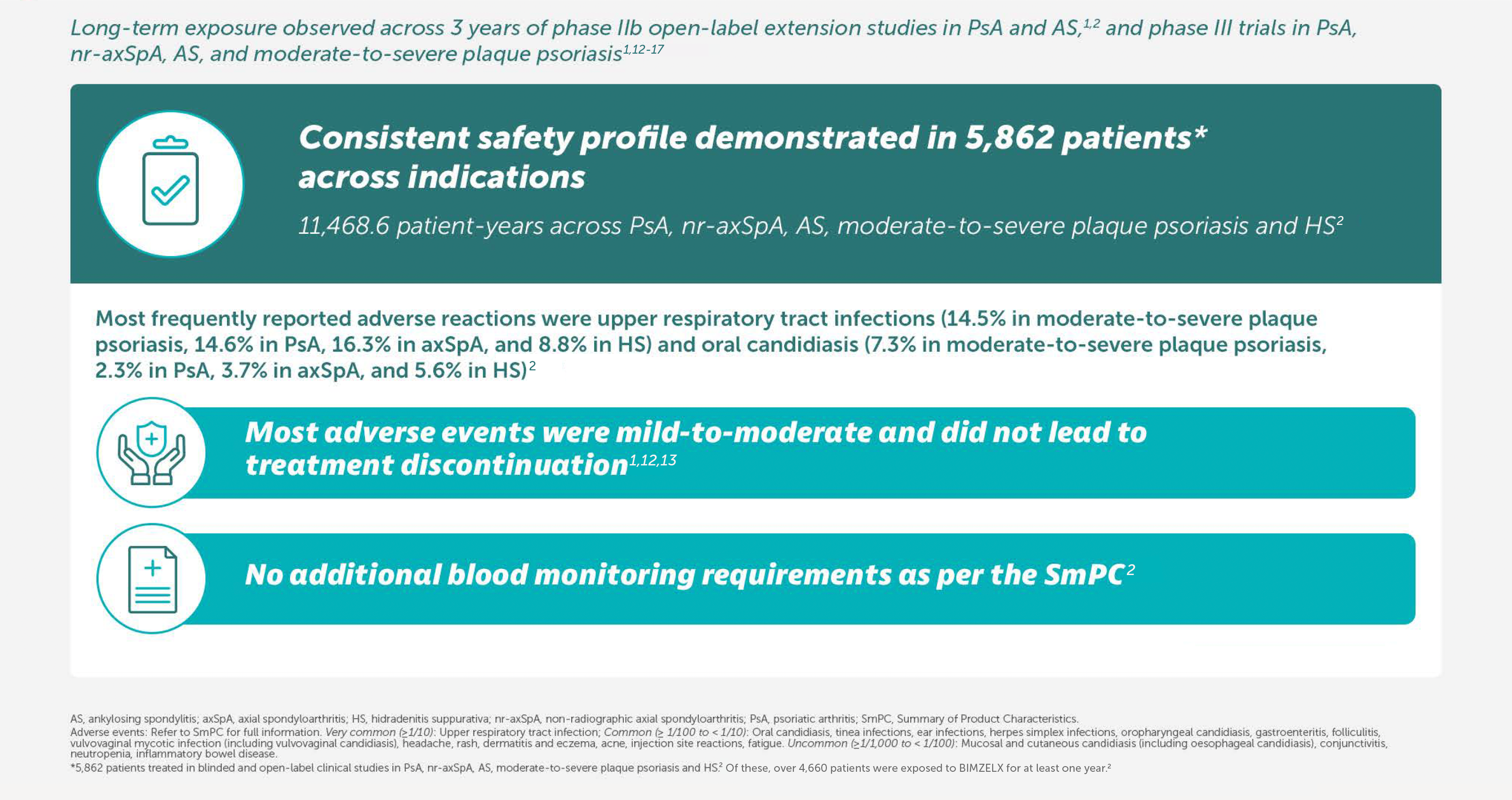
Most frequent TEAEs to Week 104 by preferred term (EAIR/100 PY) were COVID-19 Infection (13.2), nasopharyngitis (10.2), and upper respiratory tract infection (6.0),22Most frequent TEAEs to Week 104 by preferred term (EAIR/100 PY) were COVID-19 infection (13.2), nasopharayngitis (10.2), and upper respiratory tract infection (6.0).4
*Patient population is pooled and includes patients from both BE MOBILE 1 and BE MOBILE 2 who received a dose of BIMZELX, including those originally randomised to placebo, events are reported after switch only.4
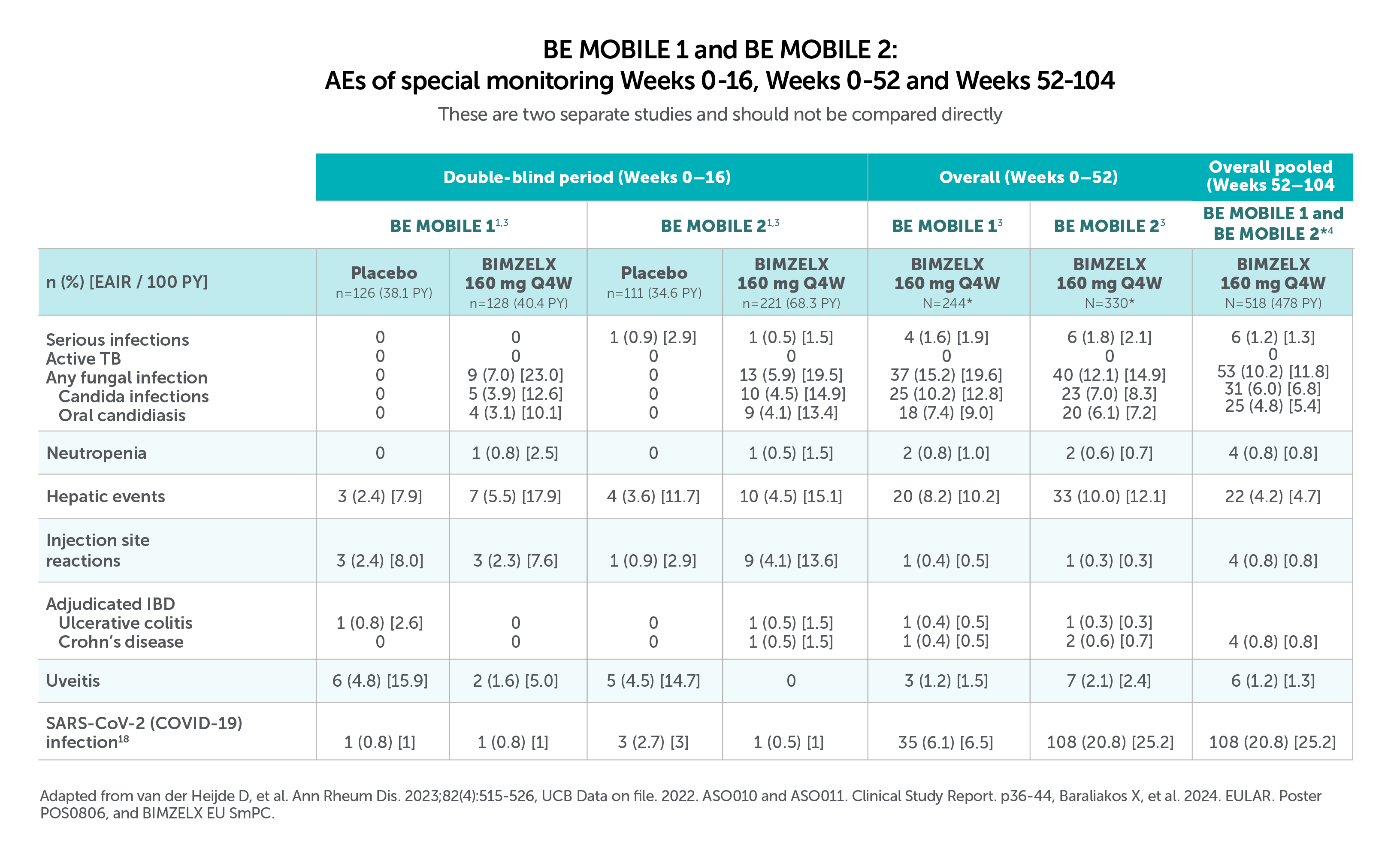
Most common TEAES (>5.0% in BE MOBILE 1 and BE MOBILE 2) with BIMZELX across 52 weeks were:
• Nasopharyngitis (12.3%, n=30/244, and 9.1%, n=30/330), oral candidiasis (7.4%, n=18/244, and 6.1%, n=20/330), and URTIS (9.4%, n=23/244, and 6.4%, n=21/330) in BE MOBILE 1 and BE MOBILE 2, respectively3
Most frequent TEAEs to Week 104 by preferred term (EAIR/100 PY) were COVID-19 Infection (13.2), nasopharyngitis (10.2), and upper respiratory tract infection (6.0),22Most frequent TEAEs to Week 104 by preferred term (EAIR/100 PY) were COVID-19 infection (13.2), nasopharayngitis (10.2), and upper respiratory tract infection (6.0).4
*Patient population is pooled and includes patients from both BE MOBILE 1 and BE MOBILE 2 who received a dose of BIMZELX, including those originally randomised to placebo, events are reported after switch only.4

How to use
SIMPLICITY 2
BIMZELX is administered as a single 160 mg subcutaneous injection once every 4 weeks*2
*The recommended dose for adult patients with axial spondyloarthritis is 160 mg (given as one subcutaneous injection) every four weeks. Consideration should be given to discontinuing treatment in patients who have shown no improvement by 16 weeks of treatment.2
Pivotal phase III study design: BE MOBILE 1
Patients with nr-axSpA (TNFi-naïve and TNFi-inadequate responders)9
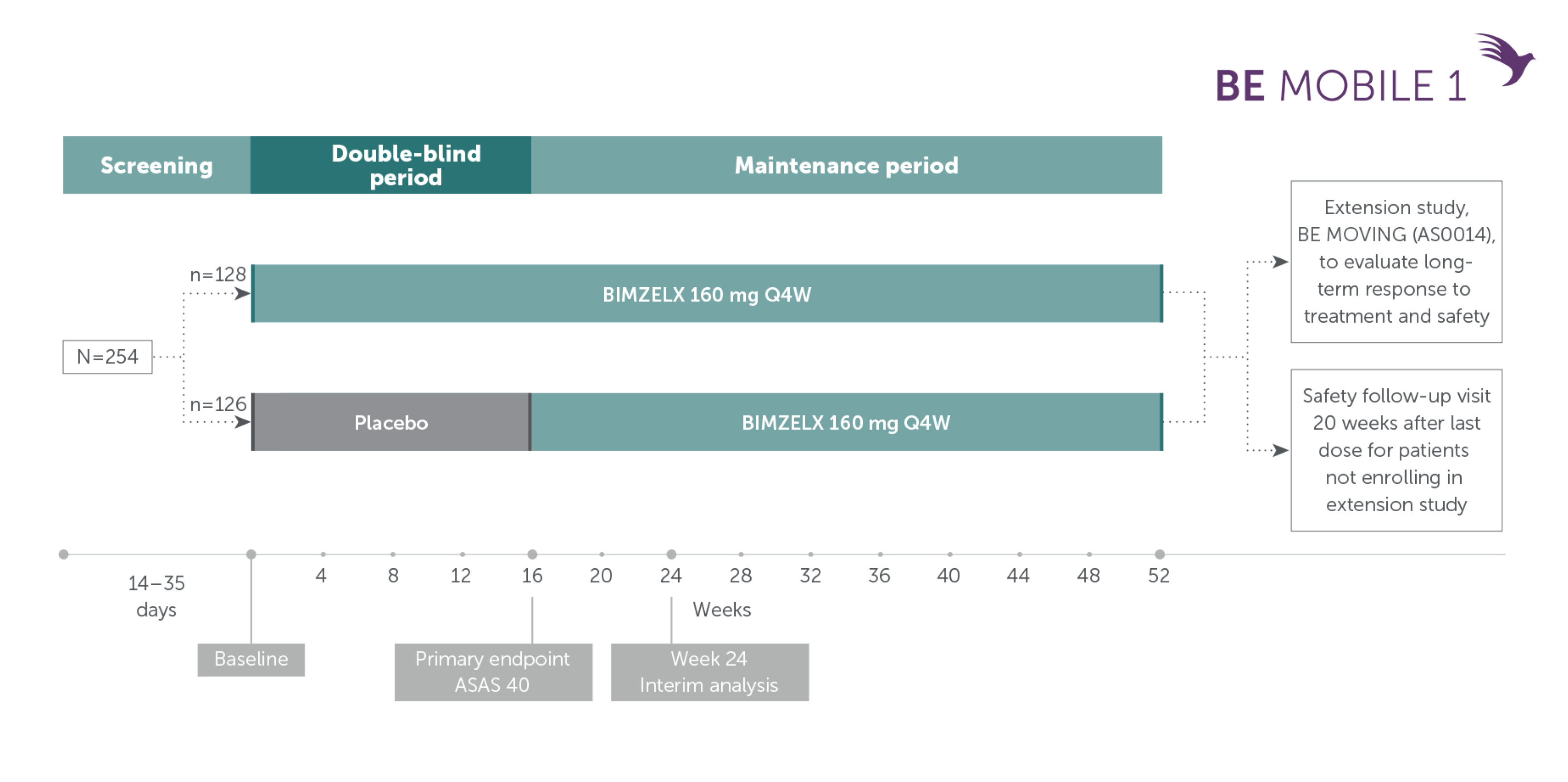
Adapted from van der Heijde D, et al. Ann Rheum Dis. 2023; 82(4):515-526. Supplementary appendix.
Pivotal phase III study design: BE MOBILE 2
Patients with AS (TNFi-naïve and TNFi-inadequate responders)9
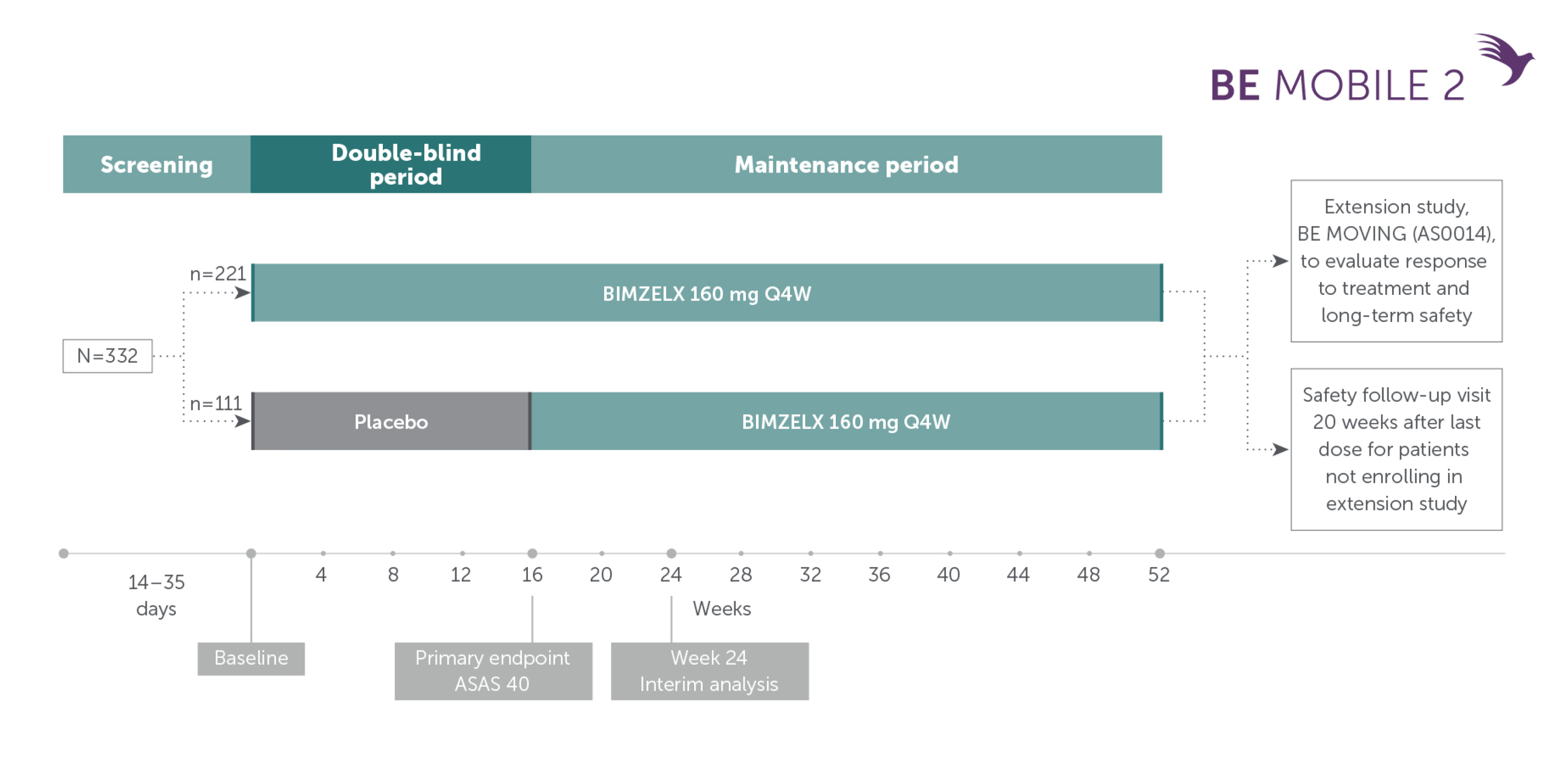
Adapted from van der Heijde D, et al. Ann Rheum Dis. 2023; 82(4):515-526. Supplementary appendix.
Phase IIb and open-label extension study design: BE AGILE
Patients with AS19
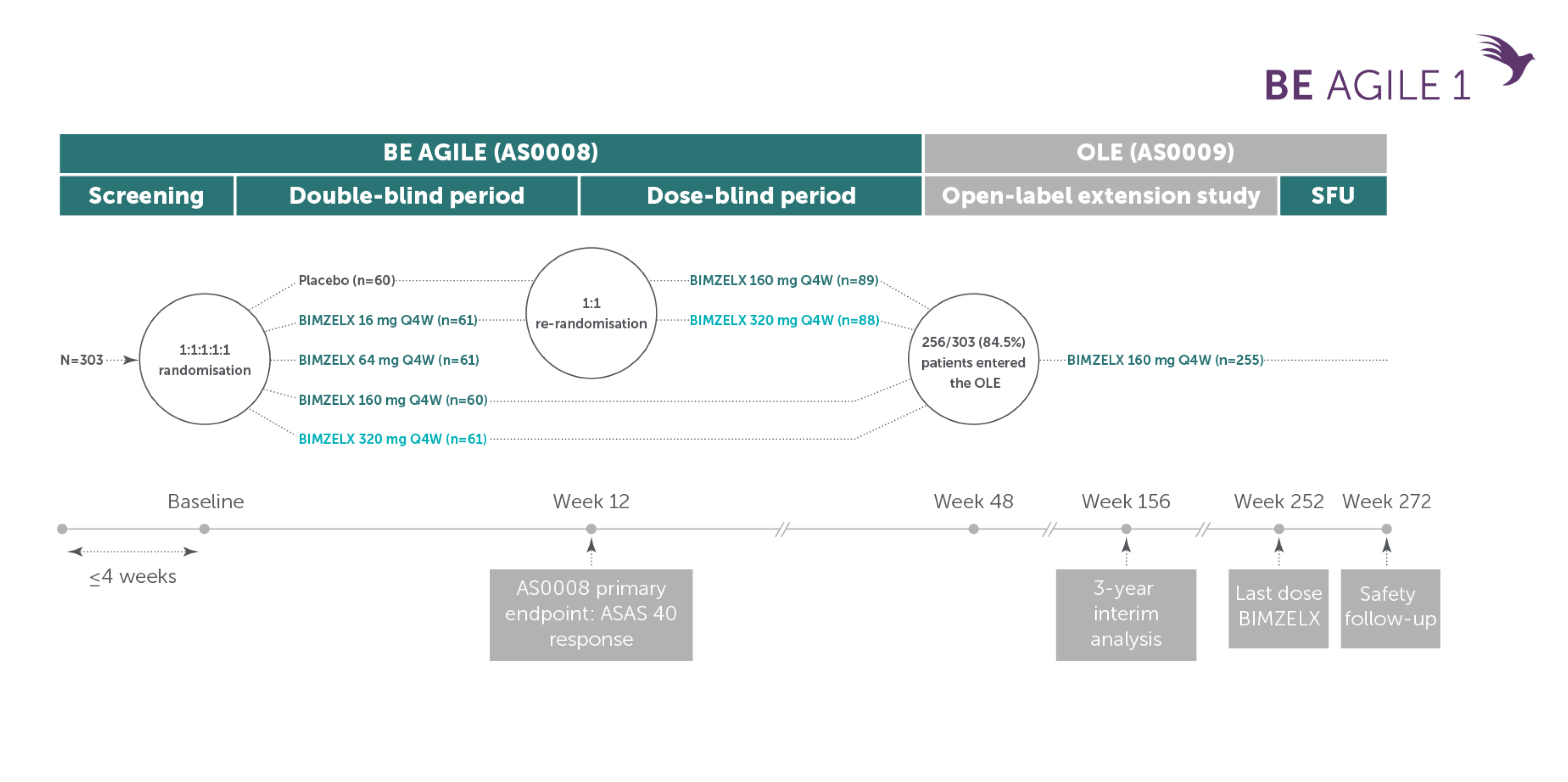
Adapted from Baraliakos X, et al. Arthritis Rheumatol. 2022; 71(q12): 1943-58. Supplementary Appendix.

IE-BK-2400125
Date of creation: September 2024