BIMZELX® (bimekizumab)▼: THE FIRST AND ONLY DUAL IL-17A AND IL-17F INHIBITOR
BIMZELX® is indicated for: moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. Bimzelx, alone or in combination with methotrexate, is indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumatic drugs (DMARDs).1 Bimzelx is now approved by the EMA and MHRA for the treatment of active moderate-to-severe HS in adults who have had an inadequate response to conventional systemic HS therapy.
BIMZELX: The opportunity for complete, fast and lasting skin clearance1,2
The Opportunity for Complete, Fast and Lasting Skin Clearance1,3
• 68.2% (n=238/349) achieved PASI 100 at Week 16 (placebo n=1/86)¥*1
• 75.9% (n=265/349) achieved PASI 75 at Week 4 (placebo n=1/86)¥* 1
• 72.6% (N=197)§ achieved and maintained PASI 100 up to 4 years3
BIMZELX®▼ (bimekizumab) was well tolerated, the most frequently reported adverse reactions were: upper respiratory tract infections and oral candidiasis . Other common reported adverse reactions include tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, rash, dermatitis, eczema, acne, injection site reactions, fatigue, and vulvovaginal mycotic infection (including vulvovaginal candidiasis).2
Please refer to the SmPC for further information.
Footnotes:
¥co-primary endpoints PASI 90 and IGA 0/1 at Week 16 were met.
*secondary endpoints
§N= modified non-responder imputation (mNRI), missing data were imputed with mNRI (patients with missing data following treatment discontinuation due to lack of efficacy or a treatment-related adverse event were counted as non-responders; multiple imputation methodology was used for other missing data).
References:
1. Gordon et al. Lancet;2021;397;10273:475-486
2. BIMZELX® (bimekizumab). Summary of Product Characteristics.
3. Strober et al. AAD 2024; oral presentation.

Consider BIMZELX for your eligible patients with moderate to severe plaque psoriasis who want the opportunity for complete, fast and lasting skin clearance‡4
‡ Complete skin clearance defined as PASI 100. Response at Week 16 in patients randomised to BIMZELX 320 mg Q4W at baseline (NRI).4
NOW ALSO LICENSED FOR USE IN PsA
Find out more about BIMZELX in psoriatic arthritis
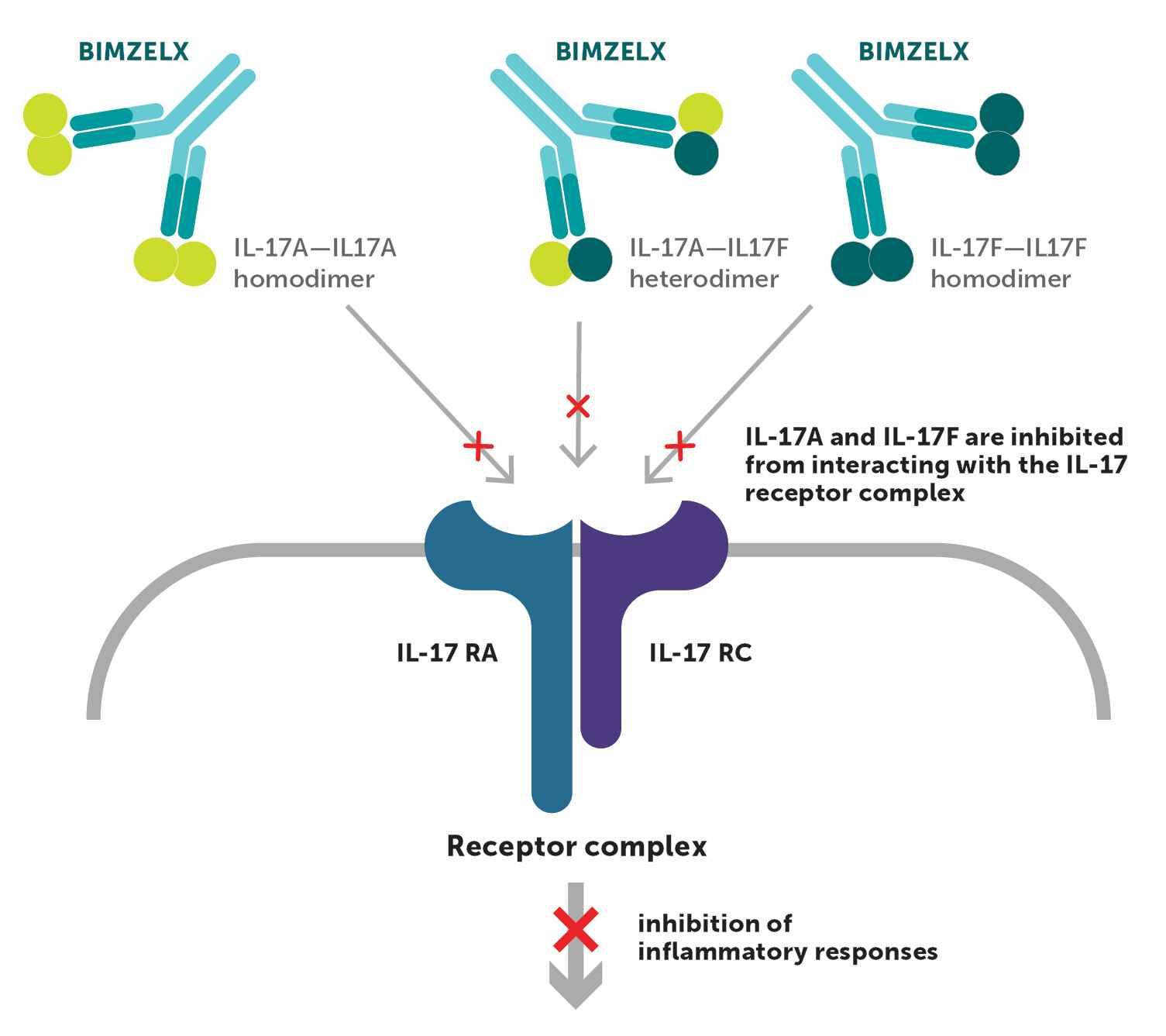
BIMZELX is the first biologic designed to selectively and directly inhibit both IL-17A and IL-17F1,3
MECHANISM OF ACTION

Date of preparation: June 2024
References
1. BIMZELX (bimekizumab) Summary of Product Characteristics.
2. Strober, B., et al. [BE BRIGHT open label extension] Br J Dermatol. 2023. 188(6): 749-759.
3. National Institute for Clinical Excellence. NICE TA723; Available online from: https://www.nice.org.uk/guidance/ta723/. Accessed November 2023.
4. Strober, B., et al. AAD 2022. Presentation 34321.
5. Gordon, K.B., et al. EADV 2022. Poster 1569.